Answer: you will need to work out how many moles are in NH3, to get this you divide the mass by NH3’s RMM (relative molecular formula) [14+ (3×1)=17] 5.00/17=0.294 moles so now that you’ve got the moles to get molecules you need to times the moles by avargado’s constant 6.022×10^23
What is ammonia (NH3)?. Ammonia is a chemical compound of… | by KAKALI GHOSH , Teacher,blogger. M.Sc chemistry. | Medium
Convert NH3 From Grams to Moles and Molecules Weight g Convert to Moles Composition of Ammonia – NH 3 Ammonia Element Mass Percent Nitrogen 14.0067g Nitrogen 14.0067g Hydrogen 3.0238g Hydrogen 3.0238g NH3 # of Atoms Hydrogen 3 Hydrogen 3 Nitrogen 1 Nitrogen 1

Source Image: chegg.com
Download Image
Convert another chemical substance Convert grams to moles Quick conversion chart of grams NH3 to mol 1 grams NH3 to mol = 0.05872 mol 10 grams NH3 to mol = 0.58718 mol 20 grams NH3 to mol = 1.17436 mol 30 grams NH3 to mol = 1.76154 mol 40 grams NH3 to mol = 2.34872 mol 50 grams NH3 to mol = 2.93591 mol 100 grams NH3 to mol = 5.87181 mol
Source Image: kgghosh1990.medium.com
Download Image
PPT – STOICHIOMETRY PowerPoint Presentation, free download – ID:2091469 6 days agoIt’s easier to work with grams, so convert the mass: 5.988 kg = 5988 g. As you already know how the grams to moles conversion work, find the number of moles: n = 5988 g / 18.015 g/mol = 332.4 mol. You can always use our grams to moles calculator to check the result! Knowing how to convert grams to moles may be helpful in numerous chemical tasks

Source Image: brainly.com
Download Image
How Many Molecules Are In 5.00 Grams Of Nh3
6 days agoIt’s easier to work with grams, so convert the mass: 5.988 kg = 5988 g. As you already know how the grams to moles conversion work, find the number of moles: n = 5988 g / 18.015 g/mol = 332.4 mol. You can always use our grams to moles calculator to check the result! Knowing how to convert grams to moles may be helpful in numerous chemical tasks 27 How many molecules are contained in 5.00 grams of NH3? (Atomic Mass H = 1.008; N = 14.01 g/mole) out of question Select one: 0 1.77 x 1023 molecules O 3.00 x 1024 molecules 0 3.60 x 1023 molecules 5.42 x 1022 molecules 6.022 x 1029 molecules bus page This problem has been solved!
How many grams of ammonia (NH3) are present in 5.0L of a 0.050M Solution? A. 5.2g B. 4.3g C. 4.3mol D. – brainly.com
To determine the number of molecules in 5.00 grams of NH3, we need to use the concept of molar mass and Avogadro’s number. 1. First, let’s calculate the molar mass of NH3. NH3 is composed of one nitrogen (N) atom and three hydrogen (H) atoms. The atomic masses of nitrogen and hydrogen are approximately 14 and 1, respectively. The number of molecules in 5.65 g of ammonia is approximately x×1023. Wha..
Source Image: askfilo.com
Download Image
IB Chemistry Mole Concept and Empirical Formula | PPT To determine the number of molecules in 5.00 grams of NH3, we need to use the concept of molar mass and Avogadro’s number. 1. First, let’s calculate the molar mass of NH3. NH3 is composed of one nitrogen (N) atom and three hydrogen (H) atoms. The atomic masses of nitrogen and hydrogen are approximately 14 and 1, respectively.

Source Image: slideshare.net
Download Image
What is ammonia (NH3)?. Ammonia is a chemical compound of… | by KAKALI GHOSH , Teacher,blogger. M.Sc chemistry. | Medium Answer: you will need to work out how many moles are in NH3, to get this you divide the mass by NH3’s RMM (relative molecular formula) [14+ (3×1)=17] 5.00/17=0.294 moles so now that you’ve got the moles to get molecules you need to times the moles by avargado’s constant 6.022×10^23

Source Image: kgghosh1990.medium.com
Download Image
PPT – STOICHIOMETRY PowerPoint Presentation, free download – ID:2091469 Convert another chemical substance Convert grams to moles Quick conversion chart of grams NH3 to mol 1 grams NH3 to mol = 0.05872 mol 10 grams NH3 to mol = 0.58718 mol 20 grams NH3 to mol = 1.17436 mol 30 grams NH3 to mol = 1.76154 mol 40 grams NH3 to mol = 2.34872 mol 50 grams NH3 to mol = 2.93591 mol 100 grams NH3 to mol = 5.87181 mol

Source Image: slideserve.com
Download Image
What is urea?. Urea is a very notable organic compound… | by KAKALI GHOSH , Teacher,blogger. M.Sc chemistry. | Medium Jul 26, 2023The following equation can be used to convert moles into molecules. M = m * 6.02214076 * 10^23 M = m ∗ 6.02214076 ∗ 1023. Where M is the total number of molecules. m is the total number of moles. To calculate the number of molecules, multiply the number of moles by 6.02214*10^23.
Source Image: kgghosh1990.medium.com
Download Image
Ammonia Stock Illustrations – 1,618 Ammonia Stock Illustrations, Vectors & Clipart – Dreamstime 6 days agoIt’s easier to work with grams, so convert the mass: 5.988 kg = 5988 g. As you already know how the grams to moles conversion work, find the number of moles: n = 5988 g / 18.015 g/mol = 332.4 mol. You can always use our grams to moles calculator to check the result! Knowing how to convert grams to moles may be helpful in numerous chemical tasks

Source Image: dreamstime.com
Download Image
Counting Atoms in a Formula – ChemSimplified 27 How many molecules are contained in 5.00 grams of NH3? (Atomic Mass H = 1.008; N = 14.01 g/mole) out of question Select one: 0 1.77 x 1023 molecules O 3.00 x 1024 molecules 0 3.60 x 1023 molecules 5.42 x 1022 molecules 6.022 x 1029 molecules bus page This problem has been solved!
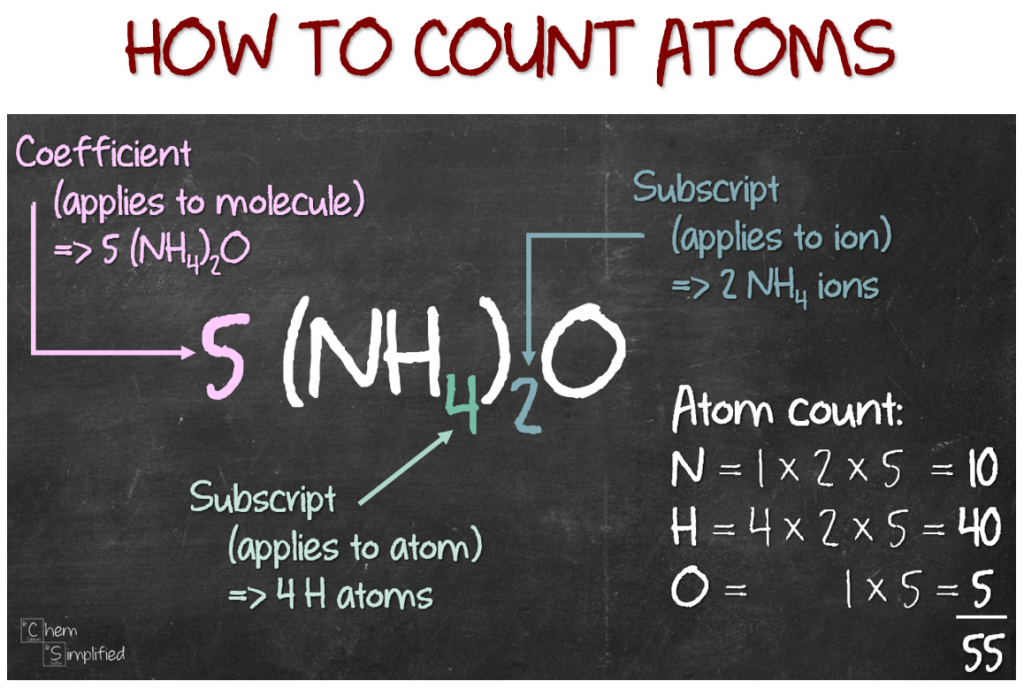
Source Image: chemsimplified.com
Download Image
IB Chemistry Mole Concept and Empirical Formula | PPT
Counting Atoms in a Formula – ChemSimplified Convert NH3 From Grams to Moles and Molecules Weight g Convert to Moles Composition of Ammonia – NH 3 Ammonia Element Mass Percent Nitrogen 14.0067g Nitrogen 14.0067g Hydrogen 3.0238g Hydrogen 3.0238g NH3 # of Atoms Hydrogen 3 Hydrogen 3 Nitrogen 1 Nitrogen 1
PPT – STOICHIOMETRY PowerPoint Presentation, free download – ID:2091469 Ammonia Stock Illustrations – 1,618 Ammonia Stock Illustrations, Vectors & Clipart – Dreamstime Jul 26, 2023The following equation can be used to convert moles into molecules. M = m * 6.02214076 * 10^23 M = m ∗ 6.02214076 ∗ 1023. Where M is the total number of molecules. m is the total number of moles. To calculate the number of molecules, multiply the number of moles by 6.02214*10^23.